Identify Which of the Following Is Soluble in Water.
Propanone Bpropane Cpropanoic acid Part B. Video Explanation Solve any question of Solutions with- Patterns of problems.

Science Experiments Solubility Of Different Substances Science Experiments Preschool Science Science Experiments Kids
Identify a water soluble ointment base from the following products.

. Right Answer is. Hence it is soluble in water. NaCl is the most soluble because it is a salt.
Identify whether the pair of substances are soluble insoluble miscible or immiscible. Iii Formic acid HCOOH has the polar group OH and can form H-bond with water. Iii Formic acid HCOOH has the polar group -OH and can form H-bond with water.
Thus formic acid is highly soluble in water. Powdered juice and Water 9. Propanoic acid Identify the compound in the following group that is most soluble in water.
Ii Toluene is water insoluble. C_ 6H_ 5OH. Then propane because both propane and ethane are non polar but propane has more Hydrogen than.
Iii Formic acid is water soluble. Thus it is highly soluble. B Ethylamine is soluble in water due to the formation of hydrogen bonds with water but aniline is insoluble since the large hydrophobic phenyl group does not allow the formation of Hydrogen-bonds.
C_ 6H_ 15 group is of non-polar nature. To rank items as equivalent overlap them. While compounds containing sulfate ions are typically soluble solubility rules state that an exception is BaSO4.
Identify the compound in the following group that is most soluble in water. Views 11K Share. Part B Identify the compound in the following group that is most soluble in water Rank the compounds from most soluble in water to least soluble in water.
Option B is correct. Ethyl Alcohol and Water 3. Using solubility guidelines which of the following ionic compounds is not properly labeled with respect to solubility in water.
Thus phenol is partially soluble in water. Iv Ethylene glycol has polar -OH group and can form H-bond. Reset Help Least soluble in water Most soluble in water octanoic acid methanolo acid oric acid pentanoic acid The correct.
Bolivianouft and 1 more users found this answer helpful. Answers 1 S Sumit. Aethanoic acid acetic acid B.
Compounds containing the hydroxide ion are usually not soluble which makes Mg OH2 insoluble and MnS is a metallic sulfide which is. So start with N aCl or sodium chloride. According to the solubility rules nitrates are soluble in water including the nitrates of silver.
Thus phenol is partially soluble in water. Iii Formic acid HCOOH has the polar group OH and can form H-bond with water. C1V1 C2V2 1ml0001L 01015M 002L C2 0250L C2 000812 Answer is B.
When you do these make sure C1 and C2 are in the same units and V1 and V2 are in the same units. Iv Ethylene glycol has polar OH group and can form Hbond. Zonrox and Water 5.
Hexanoic acid benzoic acid propanoic acid propanoic acid Identify the compound in the following group that is most soluble in water. You will need to become familiar with the solubility rules because that will help you determine what compounds are soluble and what compounds are insoluble in water. Which of the following would be soluble in water.
Thus toluene is insoluble in water. Pentane 1-hexanol propanoic acid. Identify the compound in the following group that is most soluble in water.
V Chloroform is water insoluble. Thus formic acid is highly soluble in water. MgNO₃₂ CaCO₃ PbBr₂ ZnS.
Ii Toluene C6H5CH3 has no polar groups. Solution Verified by Toppr i Phenol is partially soluble in water. That would be copper II hydroxide or CuOH2.
Is insoluble in water because it is an aromatic hydrocarbon non-polar while water is polar in nature. Primary amines contain two carbon-containing groups bonded to the nitrogen atom. Thus formic acid is highly soluble in water.
Ethanol C 2 H 5 O H forms hydrogen bonding with water. Thus toluene is insoluble in water. Thus it is.
These rules are usually based on experimental observation of diverse groups of compounds. Thus phenol is partially soluble in water. Thus toluene is insoluble in water.
However halides are soluble in water except those of silver lead and mercury. Phenol is partially soluble because phenol has a polar -OH group and aromatic phenyl C 6 H 5 - group. Hexanoic acid Coctanoic acid.
Efficascent Oil and Water 7. Fabric conditioner and Water 8. Solve any question of Alcohols Phenols and Ethers with- Patterns of problems.
Shredded Candle Wax and Water 10Water and. Ii Toluene C 6 H 5 CH 3 has no polar groups. 2-butanol because it is an alcohol.
Ii Toluene C 6 H 5-CH 3 has no polar groups. Vinegar and Soy Sauce 4. Is partiallty soluble in water due to weak dipole-dipole interactions in the molecules of pheneol and water.
C_ 7H_ 18. Vi Pentanol is partially soluble in water. Oil and Water 2.
A 3682 g sample of KClO3 is dissolved in enough water to give 375. Oil and Rubbing Alcohol 6. Cesium fluoride is soluble according to the solubility rule that all compounds of group 1 elements are soluble Cs.
Iv Ethylene glycol is water soluble. Identify the compound in the following group that is most soluble in water Match the words in the left column to the appropriate blanks in the sentences on the right Reset Help hexanoic acid Between pentanoic acid butanoic acid and hexanoic acid the most salutle of these compounds s This is due to its acidity low number of carbons high number of carbons pentanoic acid. Rank the following substances in order from most soluble in water to least soluble in water.
Best answer i Phenol C6H5OH has the polar group OH and non-polar group C6H5.

Which Solids Dissolve In Water Cool Science For Kids
Soluble Or Insoluble Lesson Science Cbse Class 6
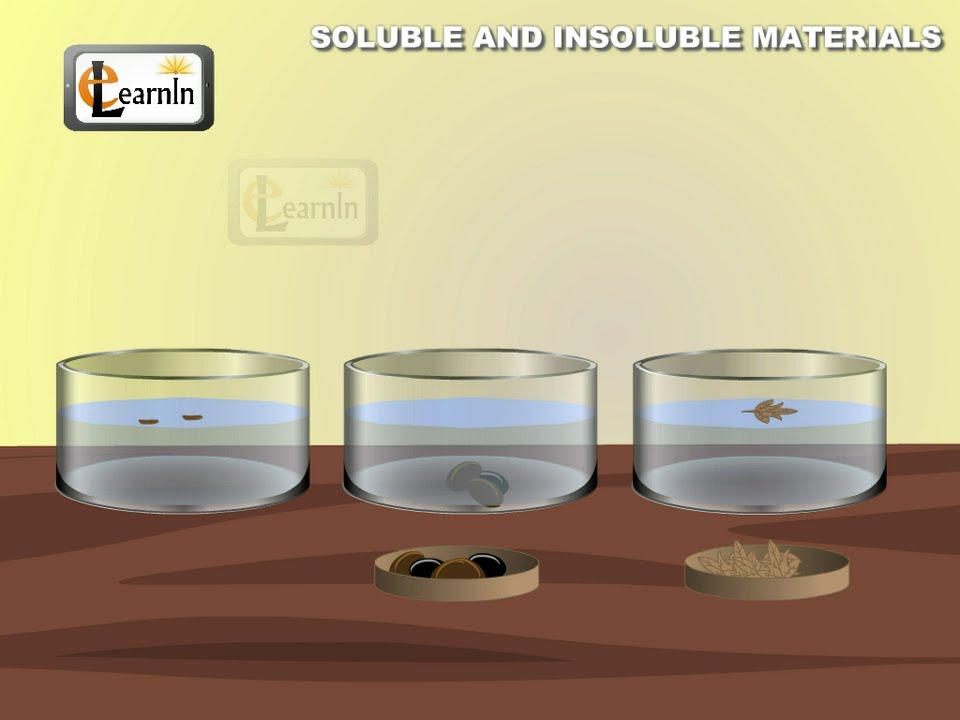
Soluble And Insoluble Materials Experiment Elementary Science Youtube

Comments
Post a Comment